By Emily & Mona

Cellular therapy is an emerging type of medicine that involves transplanting of human cells to fight disease and regenerate damaged parts of the body. Commonly, a patient’s own cells are taken and modified to have certain beneficial characteristics before being reintroduced back into their body.
Previously medicine and therapies have depended on organic compounds, many of which alleviate issues instead of resolving them completely. Using cellular therapies could potentially offer a regenerative therapy that can resolve several ongoing conditions instead of just managing them.
The most widely used cell therapy is hematopoietic stem cell transplantation,
which is used to treat cancers of the blood. A recent therapy called CAR-T cell
therapy has recently emerged as an effective treatment for certain blood cancers
and could possibly be used to treat other types of cancers and diseases too.
A brief overview of cell therapy and its product
Facts About Cellular Therapies
-
Cancer is a disease where the body’s own cells have turned against it, These traitors begin growing rapidly, stealing nutrients and invading the rest of the body. Normally, the immune system tracks and removes organisms and compounds that are damaging the body like this. However, compared to foreign invaders, cancer cells often more closely resemble the body which makes it more difficult for them to be detected. They can also hide, evade or suppress the immune cells that would usually find and destroy them.
CAR T-cell therapy gives the immune system a tool to detect the cancer cells. It takes one type of immune cell called T-cells from the patient and adds a particular gene. The gene gives it instructions to make a chimeric antigen receptor (CAR) which specifically detects a signature that is more commonly present on tumour cells, allowing the T cell to finally be able to recognise the tumour and kill it.
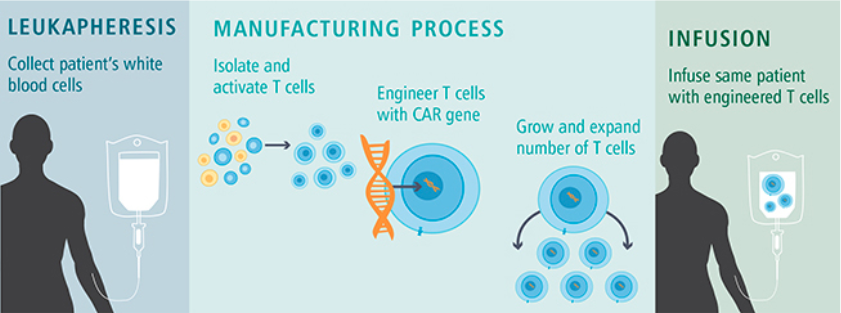
Before being treated, a patient is first evaluated through tests and screenings to determine if CAR T-cell therapy is a viable option. If they are, their T-cells are collected and re-engineered by adding a particular gene for CAR, making them CAR-T cells.
In a process which can take several weeks, the genetically altered T cells are multiplied until there are millions of them and frozen for transportation. The CAR-T cells are then transferred to a patient through a one time infusion. They are monitored for a few weeks to observe the outcomes and treat any side effects.

Current advancements
The first CAR T-cell treatment was approved in 2017, and currently CAR T-cell therapy has proven effective in treating two blood cancers: B-cell acute lymphoblastic leukaemia (ALL) and adult diffuse large B-cell lymphoma (DLBCL). These treatments are known as Tisagenlecleucel and Axicabtageneciloleucel respectively.
There are some main limitations of CAR-T cell therapy at the moment, one being that despite the high remission rates seen in patients, further research has indicated that up to 50% of patients relapse within a year of treatment. The process is also time-consuming and expensive.
At the moment, there are no approved CAR-T treatments for solid cancers.
Recent advances in CAR-T cell engineering

-
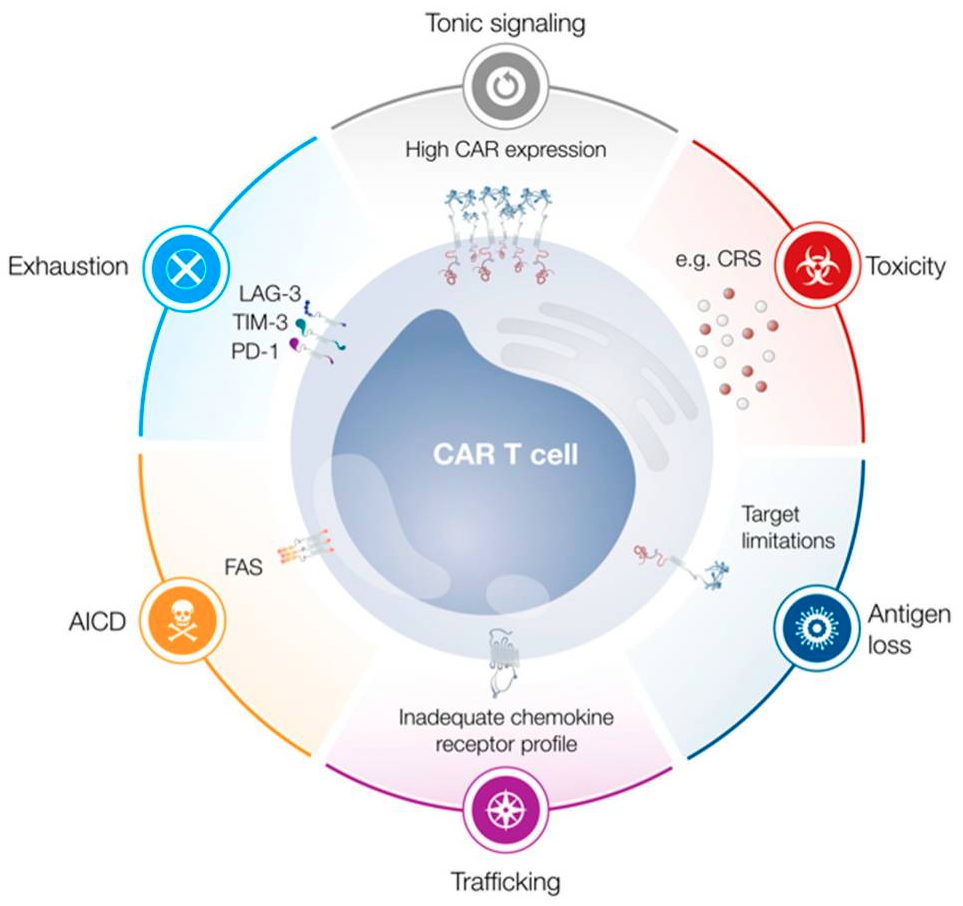
Limitations in the Design of Chimeric Antigen Receptors for Cancer Therapy
The complexities of cell therapy lead to many concerns due to limitations that arise. The main notable limitations currently are tonic signalling, activation-induced cell death (AICD) and exhaustion. These can contribute to a number of issues, such as limiting functionality, persistence and proliferation of the cells. Also, some other concerns could be difficulty of introducing these car-t cells to the target site and the triggering of cytokine release syndrome (CRS)
Cell therapy, T-Cell therapy included, relies largely on clinical trials to inform researchers on efficacy and safety. Cell therapies are extremely personalised, which makes it differ from person to person and adverse effects could be detrimental, such as cytokine storms. So, this raises ethical issues as patient safety and transparency of information to patients and potential adverse effects must be communicated clearly.
Institutions, researchers and clinicians must have a thorough framework of recruitment and proper equipment and knowledge to run such clinical trials ethically. Sourcing of cells must be ethically considered too as therapies can be autologous or allogenic and should follow the medical ethics “no-harm” principle. These cells are cultured in vitro and then reinfused into the patient so quality, tissue of origin, and other exact information must be carefully recorded. These are just a few ethical considerations needed for cellular therapies.