By Kevin Mao

Chimeric antigen receptor (CAR) T cell therapy is quickly gaining traction as a highly effective, personalised and potentially lifesaving immunotherapy for cancer treatment. By harnessing our body’s own immune defence system, CAR-T cell therapy is able to mitigate the devastating effects of autoimmunity and long-term consequences of immunosuppression observed in traditional immunotherapy administrations.
CAR-T cell therapy has yielded promising results in patients with leukaemia and lymphomas allowing for priority regulatory approval and rapid expansion of CAR-T cell clinical trials around the globe.
The prettier the roses, the sharper the thorns. CAR-T cells have overtaken the
pharmaceutical market as a strong contender to take the crown of becoming the
gold-standard in the treatment of blood borne cancers. However, the largely
uncharacterised mechanism of action, durability of response and prevalence of
serious safety concerns calls for the urgent need of ongoing review, evaluation
and implementation of stringent regulatory frameworks.
-
The Therapeutics Good Administration (TGA) is the Department of Health’s regulatory agency that regulates and oversees the safety, quality and efficacy of therapeutic goods in Australia. For cellular therapies such as CAR-T cells, there is a comprehensive review and evaluation process before the treatment is approved for use in a clinic.
The TGA seeks to adopt a similar framework as the European Medicines Agency guidelines concerning genetically modified cells. CAR-T cell therapy is classified as a class 4 biological compound with a very high safety risk and is required to undergo the Clinical Trial Exemption (CTX) scheme. In 2017 the TGA developed expedited approval pathways for specific prescription medicines akin to the FDA accelerated approval pathway harnessed by many CAR-T cell therapies now available on the market.

-
With only two CAR-T cell therapy agents currently approved in selected countries, including Australia’s Therapeutic Goods Administration announcement of Kymriah in early 2020, the exploratory journey of CAR-T cell therapy in Australia has just begun. In a recent literature review of current CAR-T cell developments, there was shown to be over 390 documented CAR-T cell clinical trials around the world, with hundreds more in preclinical development. A majority of these trials are early phase, not randomised, limited in their breadth and incredibly difficult to enrol within.
Additionally, the current clinical trial framework and vetting process has raised much concern within the community, especially regarding the validity of many advertised cellular therapies, especially CAR-T cell trials. Within the CAR-T cell trials identified in the report, only a handful were active, linked peer-review literature or even posted clinical data. The inability to link many CAR-T cell trails to a verified identifier puts not only the safety of patients seeking CAR-T cell therapy at risk, but also jeopardises the integrity of evidence based medicine and exacerbates the misinformation relating to CAR-T cell efficacy and safety.
As cellular therapies revolutionise the treatment of chronic and end stage illnesses, we as a community of patients, clinicians and citizens must remember to be sceptical, aware and display good stewardship.
Interactive map for cell & gene therapy tracking
Still in its infancy stages
As Australia rises to become a popular hub for preclinical and early stage clinical trials, with the first Australian CAR-T cell clinical trials performed at the Peter MacCallum Cancer Centre , there has been increased consideration regarding the feasibility of Australian CAR-T cell therapy development. In August 2019, Cell Therapies was accredited to be the first Australian facility licensed for commercial production of CAR-T cell therapy.
Currently approved in only the Royal Melbourne Hospital and Peter MacCallum Cancer Centre , the impact of CAR-T cell therapy on Australian’s is still quite limited. The question then becomes, do the benefits outweigh the risks? Are we willing to accept the weeks and months required to manufacture the therapies, challenges of streamlined shipping, exorbitant prices and difficulty maintaining purity, potency and consistency when there seem to be a plethora of safety and efficacy concerns?
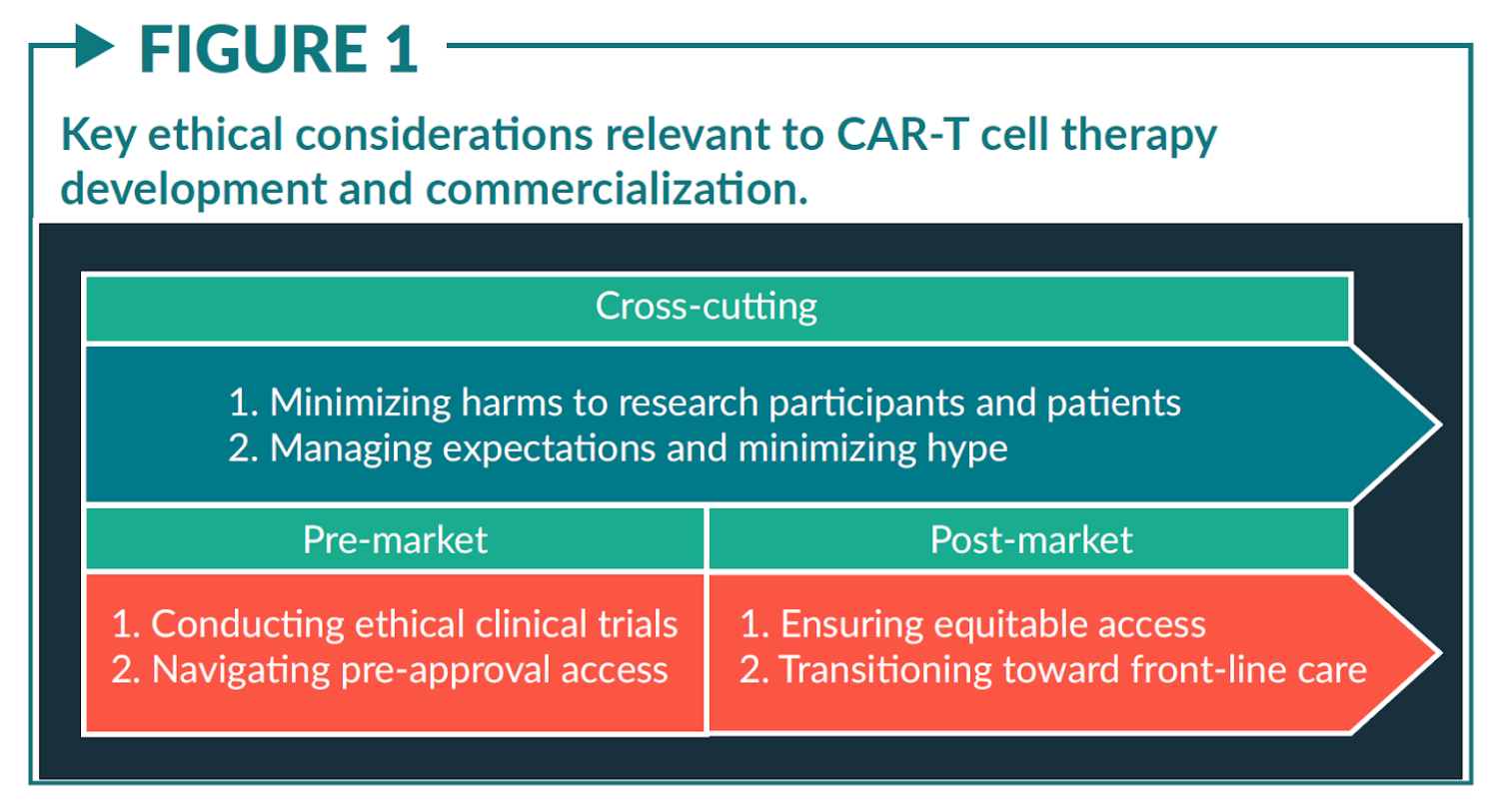
CAR-T cell therapy has proven to be productive in the treatment of certain blood cancers, however, the significant safety concerns are not to be neglected. There is a considerable frequency of off target effects leading to poorer patient outcomes due to increased susceptibility to infection, immunosuppression, toxicity and graft vs host disease. In addition to the safety concerns, the ethical considerations regarding the intellectual property and integrity of patient privacy cannot be forgotten.
On the one hand, CAR-T cells are just like any other therapy, a physical entity that treats disease. However, the story of Henrietta Lacks reminds us of the ethical and policy issues associated with biospecimen research. As such, in conjunction with further optimisation and evaluation of the scientific techniques related to the production, administration and storage of CAR-T cell therapies, we must not lose sight of our humanity.
 Credits
Credits
CAR-T cell therapy hit the world initially as the ‘magic bullet’; a new, exciting immunotherapy that showed great promise. As researchers continued to elucidate this mystery treatment, it quickly became apparent that we had no rules to govern the use of CAR-T cell therapy. Thirty years later, we have made significant progress, but we must not become complacent as there is much to be done in implementing regulations that ensure patient safety, equitable access and rigorous validation policies.
There is never a better time to hold hands, collaborate and work towards a unified framework and set of recommendations that culminates the expertise of clinicians, scientists and policymakers with the input of the public. Let us harness the power of all 194 medicines regulatory agencies across the world and through international harmonisation and consensus , ultimately benefit the patient.
