By Alex & Thanushi

CAR-T cell therapies approved for specific cancers currently cost half a million dollars per dose. What makes them so expensive and how do we make them accessible to all Australians?
-
CAR-T therapy is a new cancer treatment that is approved to treat several types of blood cancers. In clinical trials, approximately 50% of patients experience a benefit with some showing no detectable sign of the cancer in their body.
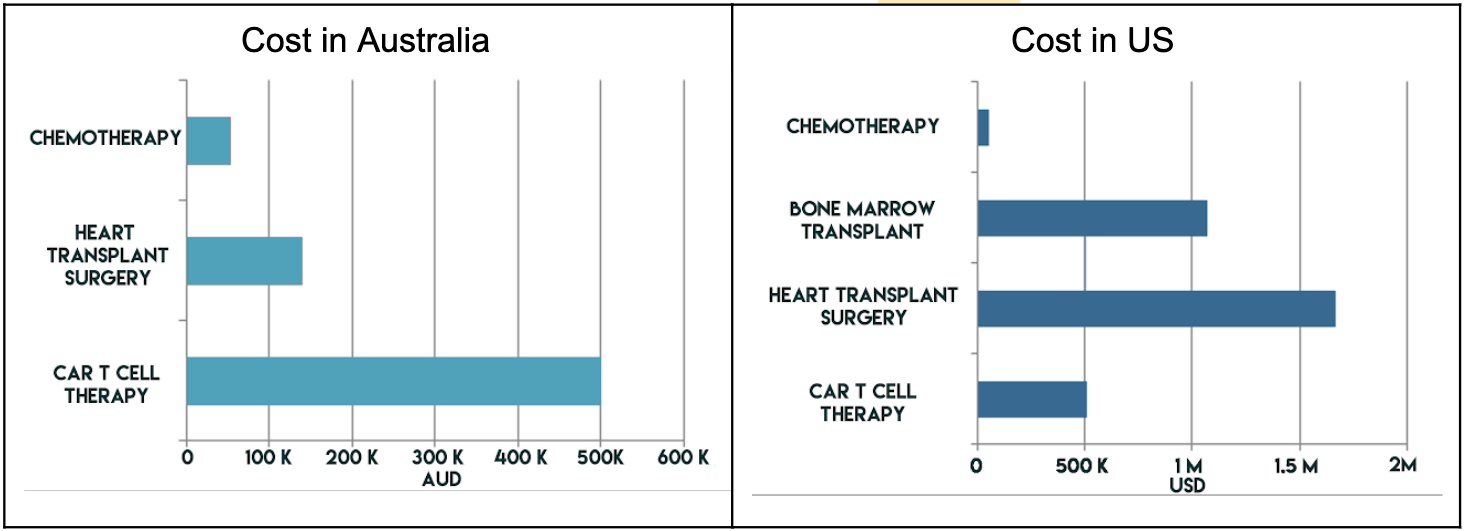
In Australia, the only currently approved CAR-T therapy is Kymriah which costs at least $500,000
for a single dose. This makes it one of the most expensive treatments in all of Australian healthcare,
considerably more expensive than the cost of heart transplant operation to a private patient
(AUD$139,900) and a chemotherapy regimen commonly used for the same types of cancers (AUD$54,297 /year).
Expanded access to cutting edge CAR T-cell therapy
Application No. 1519.1 – Tisagenlecleucel (CTL019) for treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL)
Cost of care in NSW Hospitals
Kymriah is similarly expensive at USD$511,000 whereas a donor bone marrow transplant
(another cellular therapy for blood cancers) costs USD$1,071,000. These prices are potentially
inflated due to a healthcare system that is more privatised than Australia’s but have been included
here for comparison.
ReCAP: Cost Differential of Chemotherapy for Solid Tumors
Top 10 Most Expensive Medical Procedures
Analysis determines true cost for CAR T-cell therapy
Drug pricing is contentious because pharmaceutical companies often charge a very high margin to recoup the costs of developing multiple drugs, many of which fail before one successfully comes to market.
However, even if we consider only the cost of production, Kymriah still costs approximately half a million dollars per dose in Australia. Yet, his figure does not take into account the cost of the hospital stay the patient often has to undergo after treatment or complications that may arise from treatment.
The Australian government is currently subsidising the cost of Kymriah for all eligible patients with acute lymphoblastic leukemia and specific forms of lymphoma. These patients have not responded to other treatments such as bone marrow transplants and are able to access Kymriah for free.
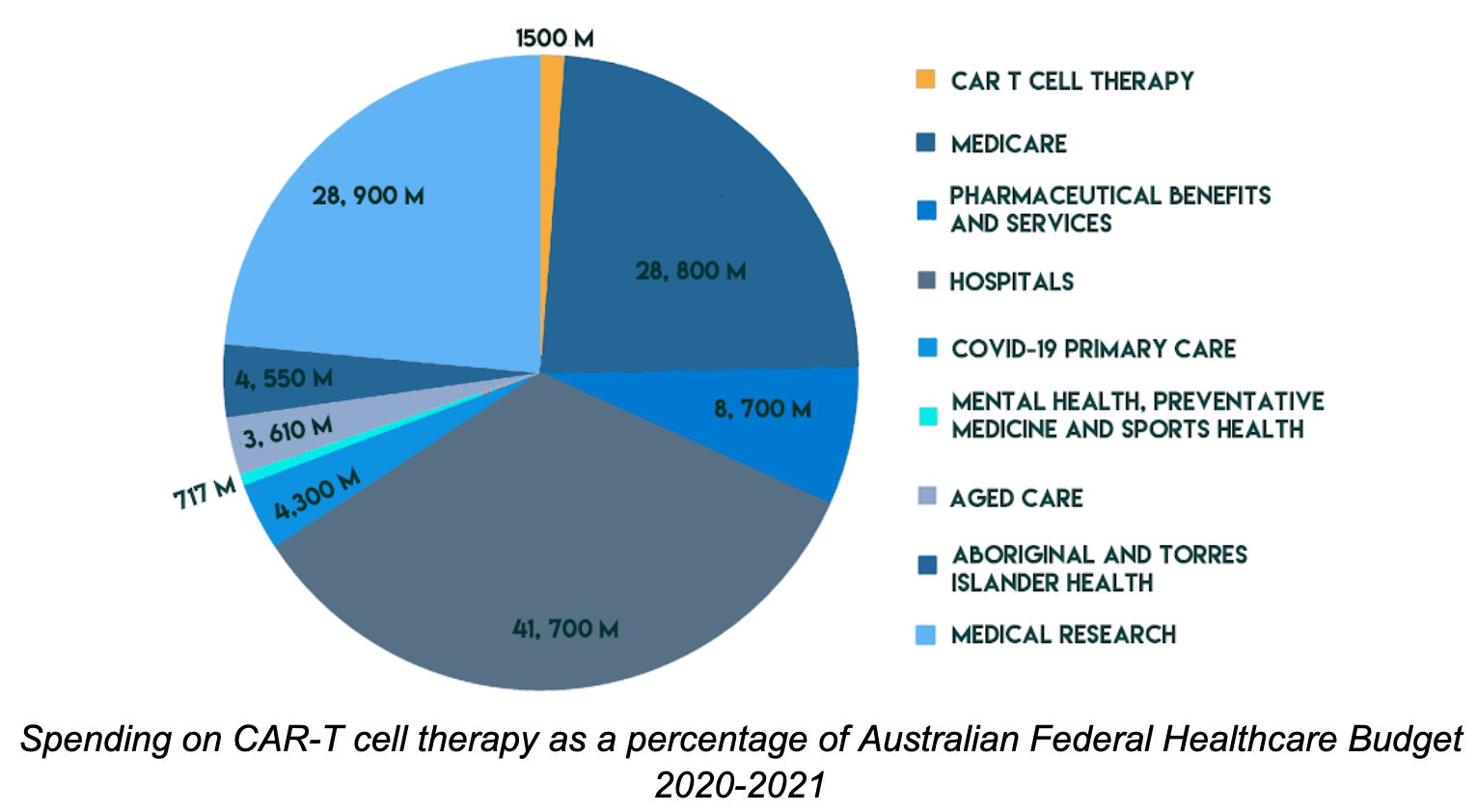
It is estimated that at least 3,000 Australians are eligible for the treatment, at a total cost of $1.5 billion. This accounts for 1.3% of the $115.5 billion Australian federal healthcare budget. Although this may seem small,the federal budget’s scope covers Medicare, public hospitals and research infrastructure for 22 million Australians so this seemingly small percentage is in reality a staggering cost for a single drug used for a small group of patients. More Info.
CAR-T therapies offer a higher survival rate and are more cost effective in the long term but they are also much more expensive than other generations of treatments More info. Given its promise in treating and even curing these three specific blood cancers, this treatment has also generated a lot of interest in being used for other types of cancers.
As CAR-T is developed to treat more diseases, will we still be able pay for it? How will we ensure it continues to be accessible to all Australians, regardless of their wealth?

-
CAR-T is a “living drug”. T cells are taken from the patient and modified, then put back in the patient to track and kill the cancer. Unlike for paracetamol or other chemotherapy drugs that are made in a single factory batch on a large scale, the CAR-T cells are grown in labs on a patient by patient basis so will be much more expensive.
There are some main limitations of CAR-T cell therapy at the moment, one being that despite the high remission rates seen in patients, further research has indicated that up to 50% of patients relapse within a year of treatment. The process is also time-consuming and expensive.
Currently, academic institutions have been mostly responsible for generating these therapies on a smaller scale for clinical trials and companies are only just beginning to manufacture them. Novartis Pharmaceuticals has just established a manufacturing location in Melbourne in partnership with Peter MacCallum Cancer Centre. Their ability to optimise this process and standardise the procedure between patients is essential to reduce the cost of the treatment. Whether or not these savings will be passed onto patients and our healthcare system is uncertain.
New technologies could also drastically change the manufacturing process to be more cost-effective. Researchers are currently exploring the possibility of developing an off the shelf CAR-T therapy. Cells from a human donor can be used to create the CAR-T cell therapy and then matched to a cancer patient who receives them. This would alleviate the need for personalised, labour intensive production and significantly reduce costs.


-
Currently, there are only 3 treatment centres certified to provide CAR T cell therapy in Australia, Royal Prince Alfred Hospital in Sydney, and Peter MacCallum Cancer Centre and Royal Melbourne Children’s Hospital in Melbourne. By partnership with Novartis, the Peter MacCallum centre is now certified to manufacture these therapies. This has vastly improved accessibility, as previously patient cells would need to be shipped to the US for manufacture.
However, patients from regional and remote areas face extra challenges in a centralised system as they must be healthy and wealthy enough to travel to these centres and so are more likely to experience treatment delays. These delays have been shown to decrease the quality of life gained from CAR-T therapy.
A balance must be maintained between having more centres closer to patients and having high quality centres. The personalised nature of the CAR-T treatment requires expert medical staff, facilities that meet manufacturing standards and the resources to deal with treatment complications which is easier to achieve in a centralised location. A reimbursement scheme to enable patients and caretakers to travel to fewer centres may prove more cost effective than setting up more centres to begin with. More info.

-
Australia’s recent approval and reimbursement of CAR T therapies will undoubtedly improve the lives of the patients that need it. Eligible patients can access these cells much faster with local manufacture, as well as for free. However, the government would have to bear a hefty cost to be able to continue to provide this reimbursement. Australia’s Therapeutic Goods Administration’s approval of Kymriah’s competitors, such as Gilead’s Yescarta, will promote innovation in manufacturing that is essential to further drive down the price.
As CAR-T is adapted to treat other cancers, more and more Australians stand to benefit from another chance at remission. Further innovation will lower costs, including future off-the-shelf treatments that would reduce the need for individualised manufacturing. For “cancer assassins” to be available to all Australians who need them, these innovations must remove the half a million dollar price.
